Behind every pharma dataset is a record of experiments, patient journeys, and scientific breakthroughs. Yet much of this knowledge is trapped in unstructured data in pharma systems that were never designed for modern analytics.
Around 80% of all data in life sciences exists in this format, making it difficult to access, analyze, and transform into usable insights. Over the past twenty years, companies have gathered large datasets without standardization, turning valuable knowledge into isolated pieces. The financial impact is just as significant.
Gartner estimates that poor data quality costs organizations an average of $12.9 million annually. As a result, even with advanced pharma data analytics, critical information often remains locked across silos, slowing R&D progress, complicating compliance, and limiting innovation.

Computools delivers pharmaceutical software development services that help organizations transform fragmented, historical datasets into connected, intelligent ecosystems. For one pharma client (under NDA), our team combined AI and ML software solutions with knowledge graph technology to unify years of experimental data, uncover hidden relationships, and enable faster, more accurate research decisions.
Recognized in IAOP’s Global Outsourcing 100 for five consecutive years, Computools brings proven technical depth and domain expertise to every pharma and healthcare project. Using advanced pharma data analytics solutions, we help companies transform complex data landscapes into strategic assets that speed up discovery, boost R&D efficiency, and enhance decision-making throughout the pharmaceutical value chain.
How we helped a Swiss pharma company transform historical lab data into strategic value
Pharma companies worldwide are under increasing pressure to extract strategic value from decades of accumulated research data. At Computools, we’ve seen this challenge firsthand and helped solve it through projects focused on data transformation, automation, and intelligent analytics. Our experience in healthcare software development services enables us to build solutions that make research data accessible, standardized, and ready for insight generation.
One of these projects involved a Switzerland-based pharmaceutical company that needed to bring order to more than a decade of laboratory experiment records, molecular reactions, compound properties, and environmental conditions stored in PDFs, Excel files, and text notes. The data was fragmented, duplicated, and lacked standard terminology, making it difficult to analyze or reuse across R&D projects.
Computools’ team applied AI in pharmaceutical research, NLP, and laboratory data optimization techniques to automatically extract key entities, unify terminology, and connect scattered datasets through a knowledge graph. This approach revealed hidden correlations between experiment protocols, reaction conditions, and molecular outcomes.
The results were transformative: redundant testing decreased by 25%, data accessibility improved by 80%, and predictive accuracy increased across R&D workflows. Scientists could identify new compound combinations and validate hypotheses much faster than before.
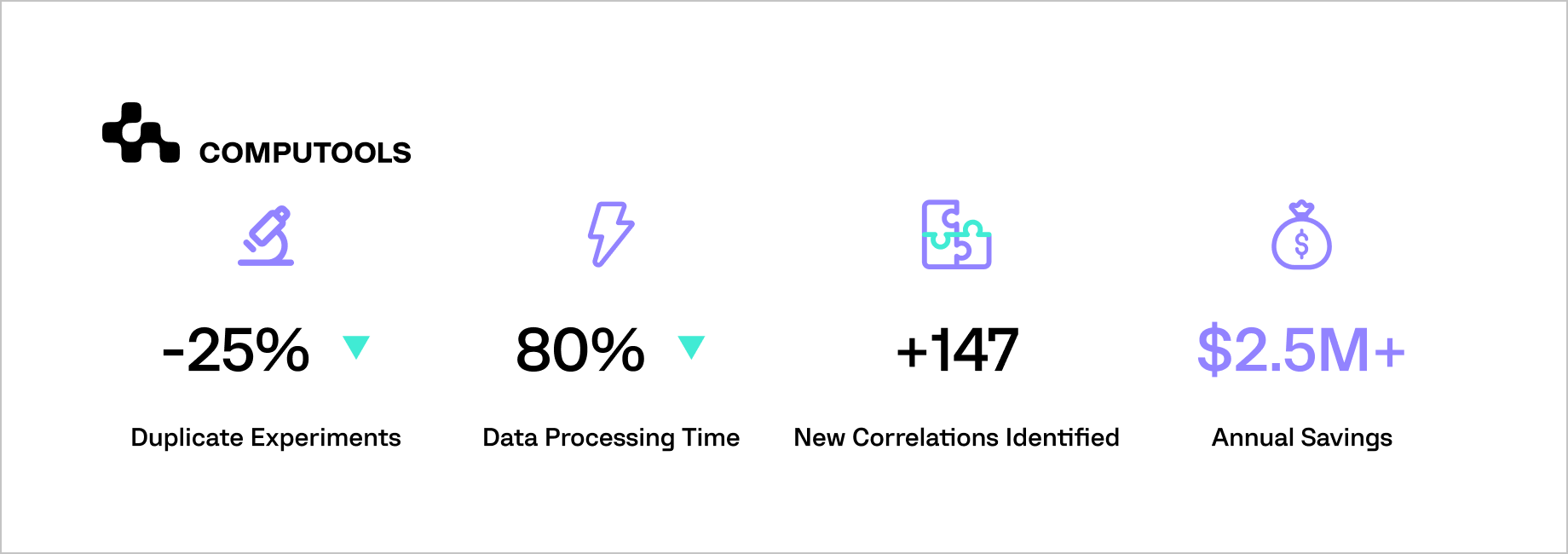
By integrating AI, ML, and knowledge graph technologies, Computools enables pharmaceutical companies to transform legacy archives into intelligent ecosystems that accelerate discovery, ensure compliance, and foster ongoing innovation.
Now, let’s explore how any pharma organization can achieve similar results and transform its historical data into a strategic advantage using analytics, AI, ML, and knowledge graphs.
10 steps to transform historical pharma data with analytics, AI, ML & knowledge graphs into strategic value
Step 1. Audit and classify historical data
The first step toward transformation is understanding and organizing what you already have. A comprehensive audit helps pharmaceutical companies locate all structured and unstructured data sources, assess their quality, and map how they connect to business goals. This creates a foundation for effective pharma data analytics, enabling teams to uncover insights hidden in years of fragmented information.
At the same time, it sets the groundwork for sustainable pharmaceutical data management, ensuring that future data collection, storage, and usage follow unified standards.
Working on a case for a Swiss pharmaceutical client, we identified more than 20 data formats across five legacy systems, including PDF experiment logs, Excel sheets, and handwritten lab notes. The lack of standardization, duplication, and missing identifiers made analysis unreliable and hindered R&D progress.
By linking each dataset to measurable KPIs such as experiment duplication rate and R&D cycle time, we helped the client turn unstructured information into a structured, high-value asset ready for advanced analytics and AI-driven decision-making.
Step 2. Cleanse and standardize data
After mapping assets, the next critical phase is data cleansing and standardization, transforming messy, inconsistent records into a trusted, analysis-ready resource. Pharmaceutical organizations often face duplicated entries, conflicting units, and terminology drift between teams or departments. Without harmonization, analytics systems produce unreliable or misleading results.
The Computools team designed an automated pipeline using natural language processing (NLP) and rule-based validation to clean our client’s decade-long experiment archive. Duplicates were removed, missing values flagged for review, and measurement systems normalized across all records. Terminology was unified through a controlled vocabulary aligned with the company’s R&D taxonomy.
This effort improved data accuracy and accelerated processing speed by 40%. The unified dataset became the foundation for predictive analytics in pharma, allowing scientists to model experimental outcomes, detect anomalies earlier, and improve repeatability across research centers.
Step 3. Build semantic models and ontologies
Once data is clean, it needs meaning. Semantic modeling gives context to information, defining what each data point represents and how it relates to others. Ontologies help connect complex entities such as molecules, reactions, and outcomes, creating a unified language for research and analytics across the organization.
Computools implemented a multi-layered ontology for our pharma case, designed to bridge laboratory, research, and clinical data domains. The model aligned with international research and experimental data standards such as OBI (Ontology for Biomedical Investigations), CHEBI (Chemical Entities of Biological Interest), and CDISC to harmonize laboratory and experimental results, while ensuring interoperability with healthcare and clinical data frameworks, including FHIR, HL7, ICD-10, SNOMED.
As a result, the company gained deeper pharmaceutical research insights, seamlessly connecting historical and newly generated data into one coherent structure—this accelerated data discovery and strengthened compliance and interoperability across the entire R&D ecosystem.
Step 4. Integrate AI and knowledge graphs
When paired with AI and graph-based reasoning, clean, structured data gains real power. Knowledge graphs represent data as interconnected nodes, allowing machines to detect patterns invisible to tabular analytics. For pharmaceutical R&D, this unlocks a new level of discovery, connecting experiments, outcomes, and historical data into a single, intelligent network.
Computools built an AI-powered knowledge graph linking years of experimental data for our Swiss client. Using machine learning, the system detected over 140 new correlations between reaction conditions and molecular behavior. This approach elevated the company’s operations from simple data analysis to a data-driven pharma strategy, supporting smarter decisions across research, compliance, and product development.
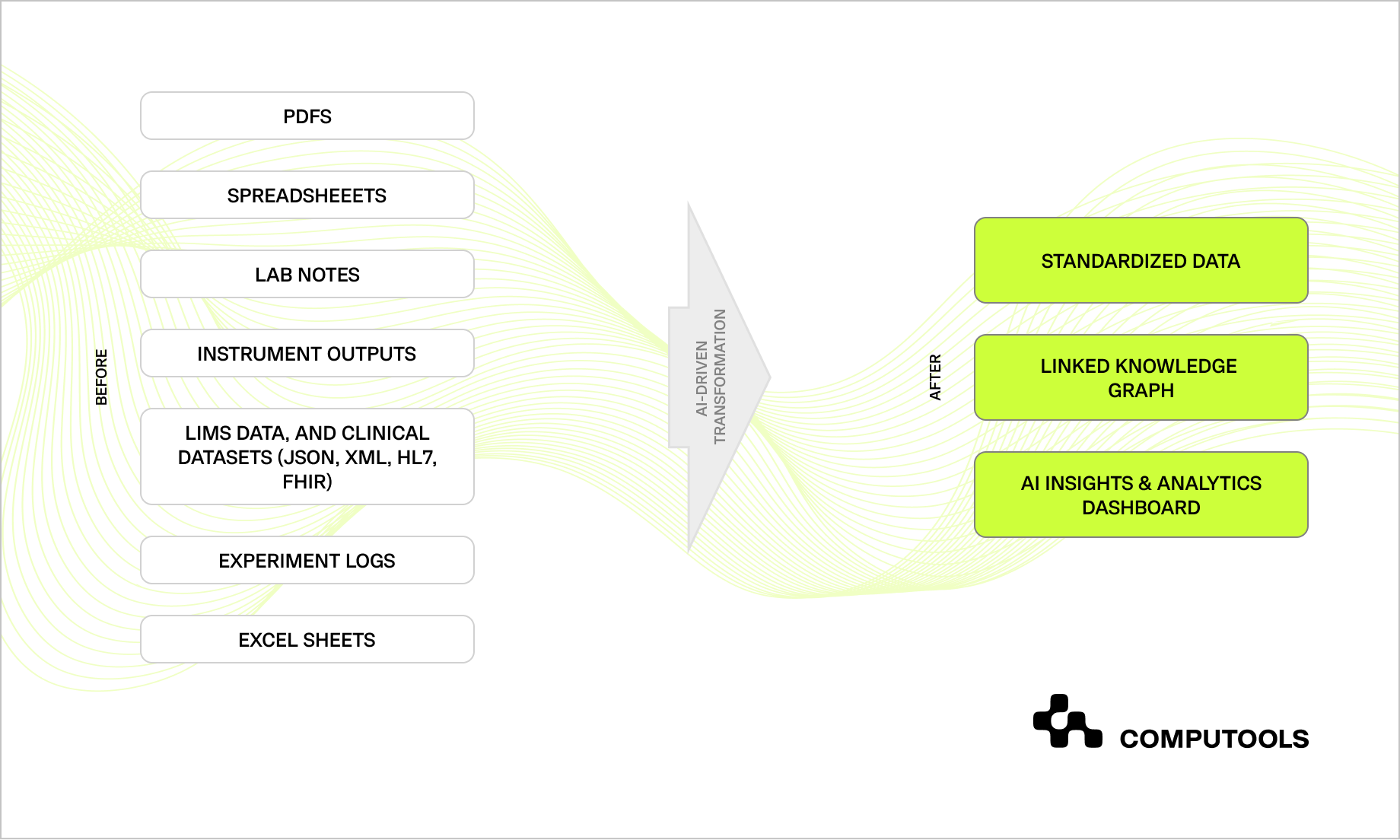
Step 5. Enable real-time analytics and visualization
The next step is making insights visible and actionable. Dashboards and interactive analytics tools transform raw metrics into intuitive visual narratives. Scientists, data managers, and executives can monitor progress, identify inefficiencies, and confidently predict next steps.
Computools implemented a modular analytics dashboard displaying experiment timelines, reaction success rates, and material usage trends. The client could instantly visualize ongoing research and historical context with these tools.
These visual interfaces turned static data into data-driven decisions in pharma, helping teams react faster, plan experiments efficiently, and justify investments based on quantifiable results.
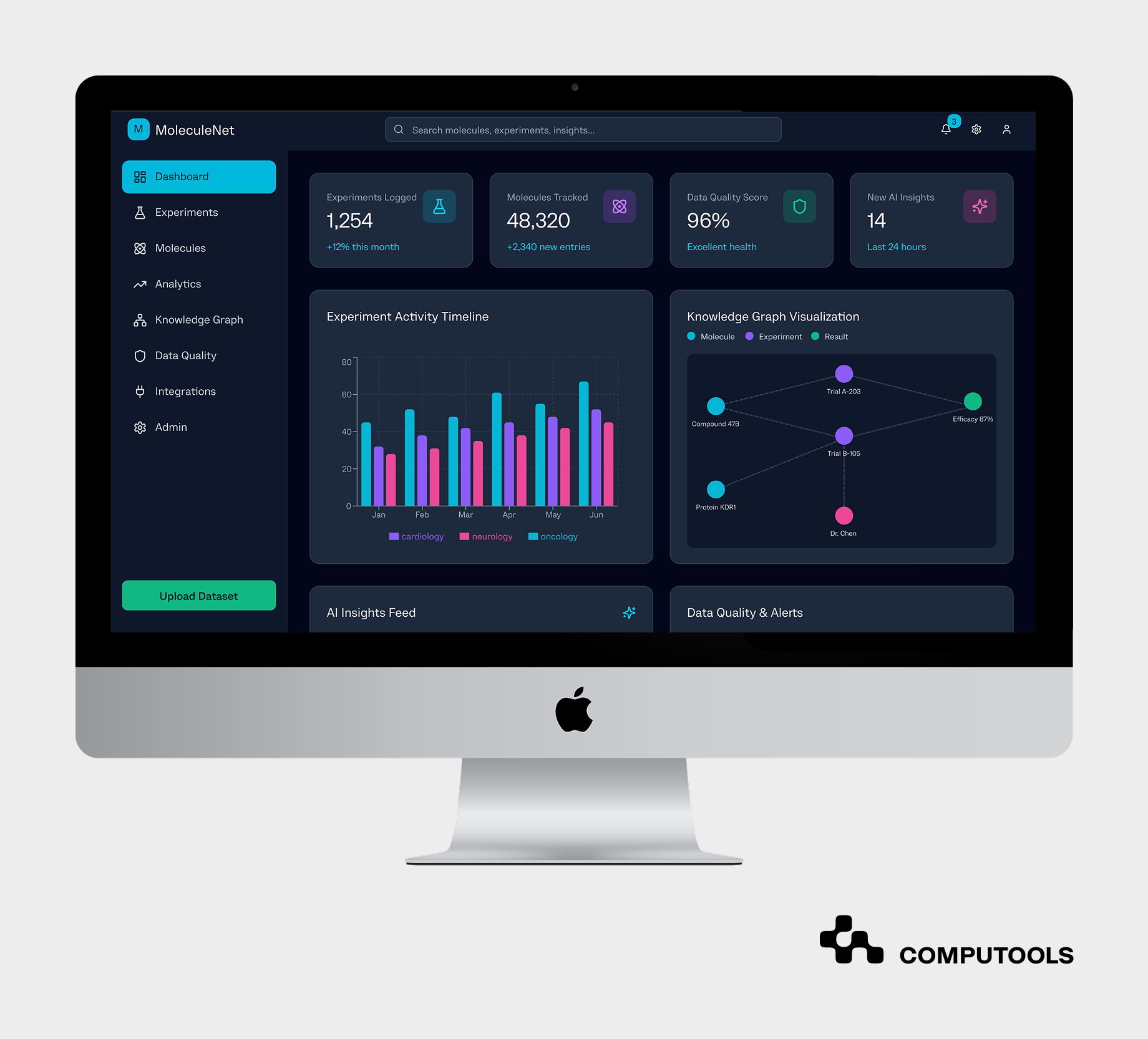
Step 6. Apply advanced analytics for forecasting
Predicting what will happen next is the real value of data maturity. By integrating machine learning and statistical models, pharma companies can forecast outcomes, identify optimal conditions, and prevent costly failures.
For the Swiss client, Computools deployed regression and clustering algorithms to forecast experiment success rates based on variables such as temperature, catalyst type, and molecular weight. The models achieved an accuracy improvement of over 30% compared to manual estimation.
This transition marked the move toward advanced analytics in pharma industry, using data to describe the past and to shape future research strategies confidently.
Step 7. Combine AI and big data for deeper research
Modern pharmaceutical discovery thrives on scale. Integrating AI and big data for pharma research allows companies to simultaneously analyze millions of data points, from genomic information to reaction outcomes, revealing previously unseen insights.
Computools extended the client’s platform with distributed ML pipelines capable of processing massive lab archives in parallel. The system identified recurring molecular behaviors correlated with specific catalysts, suggesting new candidate compounds. What once took analysts months of manual review was now achieved in hours.
This large-scale analysis accelerated the discovery pipeline and reduced the need for redundant experiments, ultimately driving efficiency and innovation.
Step 8. Generate continuous insights and governance
Once data pipelines and AI models are operational, maintaining their quality and relevance becomes essential. Continuous monitoring ensures the system evolves with new data, regulations, and business needs.
Computools implemented automated validation scripts and governance dashboards that tracked data freshness, source reliability, and AI performance metrics. This framework provided constant visibility into information flow and compliance levels, supporting pharmaceutical data management and insights at scale.
As a result, the client’s R&D and compliance teams gained confidence that decisions were always based on verified, up-to-date information.

Step 9. Embed intelligence into strategic decisions
To unlock full business value, insights must integrate directly into executive and operational workflows. This phase transforms analytics from a research tool into a decision-making engine.
Computools ensured that model-driven forecasts guided strategic planning from funding allocation to experimental prioritization by embedding AI dashboards and automated reporting into the client’s management system.
This operational shift embodied data-driven decision making in pharmaceuticals, empowering leadership to evaluate opportunities based on evidence rather than assumption and ensuring alignment between data science and corporate strategy.
Step 10. Scale predictive capabilities across the enterprise
The final phase focuses on extending these capabilities beyond R&D into the broader organization. Predictive models and analytics pipelines can inform manufacturing, quality control, supply chain optimization, and market forecasting.
Computools helped the client expand its AI ecosystem to production and logistics, forecasting reagent needs and anticipating material shortages. These capabilities transformed predictive science into an enterprise-wide advantage.
This scaling phase represents the full maturity of predictive analytics for pharma businesses, where data intelligence operates continuously across every company layer, turning innovation into measurable performance and long-term strategic value.
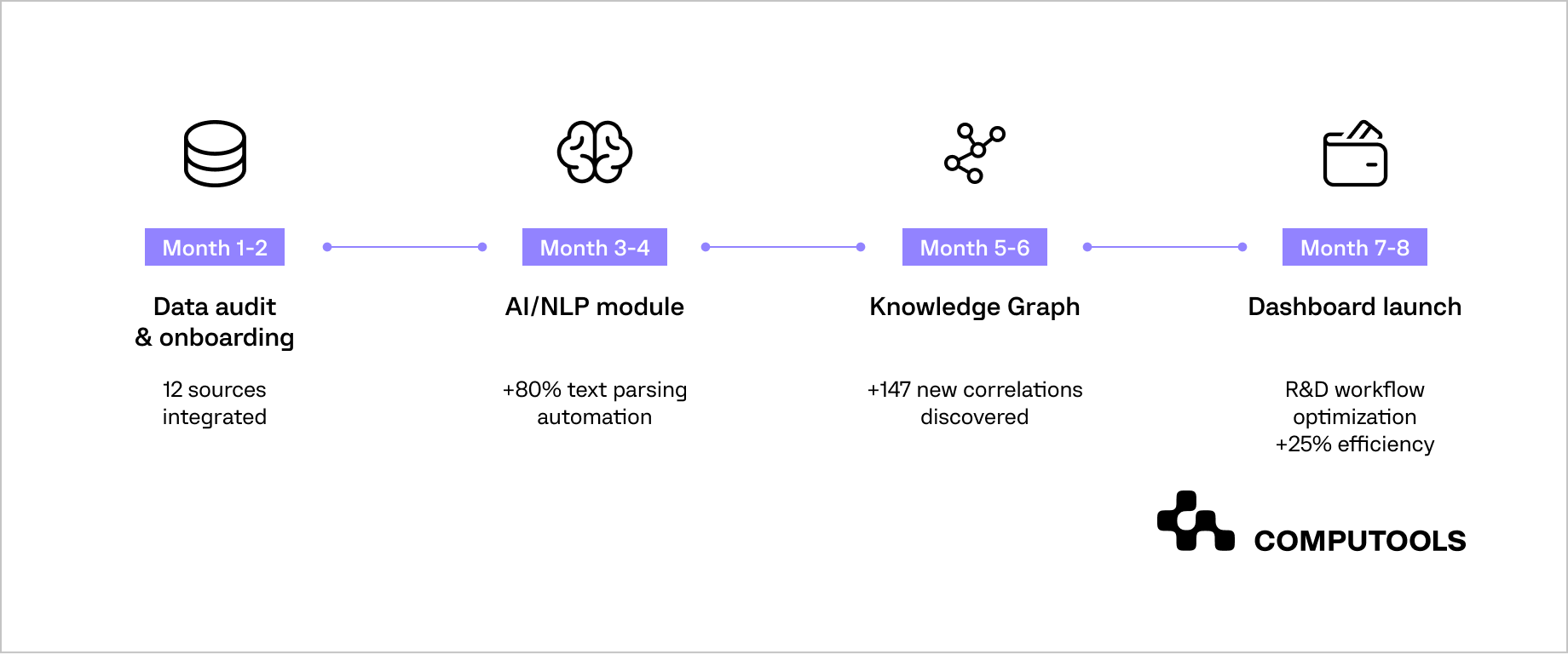
With all ten steps completed, pharmaceutical companies reach a point where data is no longer just an operational asset; it becomes the foundation for intelligent growth. At this stage, pharma data analytics shifts from retrospective reporting to a predictive and prescriptive engine that drives innovation.
The next frontier involves using AI to boost this value, turning analytics into self-driving, insight-focused systems that reshape how pharma finds, develops, and provides new therapies.
Pharma isn’t the only industry transformed by smart data; healthcare also uses AI to improve patient care and research. Read how generative AI drives change in healthcare.

Unlock up to 80% more strategic potential hidden in your historical pharma data with AI, ML, and Knowledge Graphs.
How AI is transforming the pharmaceutical industry in 2025
Once pharma companies have cleaned, structured, and connected their historical data, the next step is to use AI to generate real strategic value. What began as scattered databases and legacy systems now serve as a foundation for predictive insights, faster R&D, and more accurate decision-making.
After years of limited experiments, AI has finally moved from pilot projects into the core of pharmaceutical innovation. With rising R&D costs, tight regulatory oversight, and a growing need for personalized therapies, artificial intelligence has become the engine that drives progress, connecting analytics, machine learning, and knowledge graphs into one intelligent ecosystem.
In 2025 and beyond, AI will be a tool for pharma and the key to transforming data into discovery and discovery into measurable business value.
According to McKinsey, generative AI alone could unlock $60–110 billion in annual value for the global pharmaceutical sector. Realizing this potential requires more than sophisticated modeling; it depends on the foundation built beneath it.
That foundation is made of three essential pillars:
• Healthcare data standardization unifies insights across fragmented systems and clinical environments.
• Clinical trial data integration, connecting researchers, regulators, and manufacturers in real time to accelerate discovery and validation.
• Pharma big data solutions, ensuring scalability, security, and compliance from laboratory to market.
These pillars transform pharmaceutical data ecosystems into smart engines that drive faster R&D, better decision-making, and long-term innovation.
Where AI creates real impact
AI is transforming every process in the pharmaceutical value chain, from molecule design to market delivery. It is a functioning system that delivers measurable outcomes at scale.
• Drug Discovery: With machine learning drug discovery models, scientists can simulate molecular interactions, predict compound behavior, and prioritize promising candidates, cutting design cycles from years to weeks.
• Clinical Trials: Predictive analytics and digital twin simulations help optimize protocols and reduce costs and timelines in some cases by up to 70% and 80% respectively.
• Precision Medicine: AI interprets genomic and EHR data to match patients with personalized treatment paths, improving outcomes and adherence.
• Pharmacovigilance: The rise of NLP in pharma allows real-time monitoring of clinician notes, reports, and social data to detect adverse reactions earlier and strengthen compliance.
• Supply Chain: Predictive forecasting minimizes waste, prevents stockouts, and ensures timely delivery from lab to market.
From pilots to enterprise ecosystems
Forward-thinking companies are moving beyond isolated AI pilots to build enterprise-wide ecosystems that connect every data source and decision. Computools helps pharma enterprises evolve from fragmented silos into integrated intelligent frameworks, where pharma data analytics, machine learning, and knowledge graphs operate as one cohesive network.
This enables:
• Standardized data integration across R&D, clinical, and manufacturing systems.
• Real-time insight flows into both strategic and operational decisions.
• Built-in compliance, scalability, and ROI at every level.
Pharma data analytics in action: Real-world success stories
Global leaders are proving how pharma data analytics turns innovation into measurable results. AI is driving faster discovery, smarter trials, and more substantial ROI.
• Pfizer used AI-driven modeling and predictive analytics to accelerate the development of Paxlovid, cutting drug discovery timelines from years to months.
• In partnership with BenevolentAI, AstraZeneca applied data analytics to optimize clinical trials, improve recruitment, and identify new therapeutic targets.
• Insilico Medicine leveraged generative AI to design novel molecules, bringing its antifibrotic drug to Phase 2 trials in under 30 months.
• With Microsoft, Novartis built predictive models that forecasted trial success and guided R&D investment decisions.
These examples reveal one clear trend: when analytics, AI, and data governance work together, pharma organizations gain speed, precision, and scalability across the entire value chain.
Key challenges and strategic benefits of data transformation in pharma
The table below outlines the main challenges of working with historical pharma and the benefits of transforming it through analytics and AI-driven approaches.
| Benefit | Challenge | Solution example (Computools case) |
| Faster R&D cycles | Legacy software systems and inconsistent lab data formats slow discovery. | Integrated data from 20+ sources into one analytics platform, enabling structured insights and real-time access. |
| Smarter decision-making | Poor data quality and missing context lead to unreliable insights. | Applied NLP and pharma knowledge graphs to connect experiment data, uncover hidden patterns, and improve prediction accuracy. |
| Operational efficiency | Manual data handling increases time and cost. | Automated 80% of text parsing and standardized terminology across historical lab records. |
| Compliance and traceability | Fragmented datasets complicate audit readiness and validation. | Built unified data governance and metadata layers for full regulatory visibility. |
| Innovation scalability | Hard to replicate AI success beyond pilot projects. | Designed a modular architecture supporting continuous analytics, machine learning, and scalable model training. |
If you’re interested in how data and analytics shape other industries, explore how marketplace platforms evolve to meet new customer demands in our article 10 Best Marketplace App Development Companies for Retailers in 2025.
How to find the right partner for digital transformation in pharma
Turning historical pharma data into intelligent, compliant, and scalable systems requires more than good technology; it requires the right expertise. A trusted partner must understand how analytics, AI, and regulatory standards intersect across the pharmaceutical value chain.
When selecting a technology provider, pharma leaders should look for a team experienced in:
• AI development services that go beyond prototypes, delivering measurable ROI and production-ready intelligence.
• End-to-end pharmaceutical software development, from laboratory automation and clinical trial systems to regulatory compliance and supply chain analytics.
• Healthcare data standardization and interoperability with FHIR, HL7, and SNOMED to ensure global consistency.
• Proven quality and security credentials such as ISO 9001 and ISO 27001, ensuring every solution is safe, auditable, and scalable.
Computools combines these strengths to help pharmaceutical enterprises move from fragmented systems to unified, data-driven ecosystems. Our AI-powered platforms enable innovative R&D, faster drug lifecycle management, and seamless collaboration between scientists, regulators, and manufacturers.
With over 250 engineers, more than 20 successful healthcare and pharma projects, and recognition in the IAOP Global Outsourcing 100, Computools stands out as a partner that drives measurable transformation.
Ready to transform your pharma data? Contact us info@computools.com.
Computools
Software Solutions
Computools is an IT consulting and software development company that delivers innovative solutions to help businesses unlock tomorrow.









“Computools was selected through an RFP process. They were shortlisted and selected from between 5 other suppliers. Computools has worked thoroughly and timely to solve all security issues and launch as agreed. Their expertise is impressive.”